Nuclear spectrometry
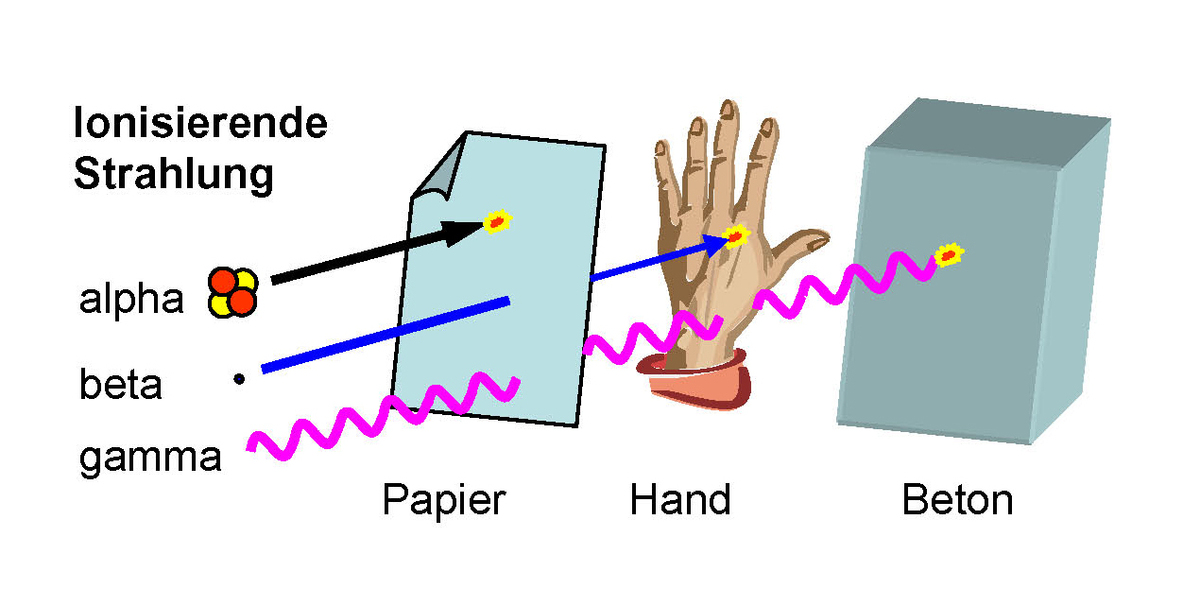
INE operates a laboratory with state-of-the-art nuclear spectroscopic methods for the analysis of alpha-, beta- and gamma emitters (Fig. 1). Fig. 2 shows the future (Hidex Ulla Counter) and the past (Packard Quantulus 1220) of low-level liquid scintillation counting. These counters are used, e.g., to determine 55Fe, 63Ni and 90Sr for waste declaration and nuclide vectors.
Nuclear spectroscopy methods:
- radiochemical separations
- α-spectrometry
- liquid scintillation counting (standard and low-level)
- γ-spectrometry
For the analysis of radioactive samples see also mass spectrometry and routine methods.
Contact:
|
+49 721 608 24747 |
Mass spectrometry
Triple-Quadrupole-ICP-MS
A triple quadrupole ICP-MS is a “tandem mass spectrometer” in which three quadrupoles Q1, Q2 and Q3 are arranged in a linear array between the inductively coupled plasma (ICP) and the detector. The two quadrupoles Q1 and Q3 are mass filters (selective) and the quadrupole Q2 (non-selective) is a collision/reaction cell (CRC). Ions of a certain mass can be selected before and after the CRC. A collision or specific reaction can be carried out in the CRC to separate interfering isobars from the analyte ions. This arrangement enables lower detection limits than single-quadrupole ICP-MS, especially for complex matrices in nuclear samples and for challenging isotopes (e.g. 129I).
Accelerator mass spectrometry (AMS)
For experiments requiring detection sensitivity at or below the fg levels, we apply and develop analytical methods using Accelerator Mass Spectrometry (AMS). This technique is not intended for routine analysis but it is dedicated to chosen studies addressing the geochemical and environmental behavior of rare, long-lived radionuclides, like 236U, 237Np, 239,240,242,244Pu and 241,243Am. AMS can be described as two mass spectrometers linked by an electrostatic tandem accelerator, providing the ions with kinetic energy up to several MeV and an effective suppression of molecular, and in some cases atomic, isobaric background through different filtering devices. Dedicated chemical separations and sample preparation procedures are required to obtain the final AMS sample. For example, for the analysis of actinides at the ultra-trace levels an iron hydroxide co-precipitation is carried out followed by the AMS target preparation as iron oxide (Fig. 2). For AMS investigations, we currently collaborate with two AMS facilities, namely the Vienna Environmental Research Accelerator (VERA) at the University of Vienna and the Ion Beam Physics group of the ETH (Zurich). One major application of the extreme sensitivity of AMS is conducted in the frame of in-situ radionuclide tracer tests performed at the Grimsel Test Site (GTS), (CH) [1, 2, 3]. Another relevant application in the safety of nuclear waste disposal involves the study of 233U and 243Am diffusion through Opalinus clay down to the ultra-trace levels [4, 5]. Furthermore, the extreme sensitivity of AMS can allow investigating the partitioning of rare, long-lived radionuclides among the various compartment of the biosphere.
Currently a new AMS laboratory is implemented at KIT-INE.
Equipment:
- Quadrupol-ICP-MS, Kollisions-/Reaktionszelle (radioaktiv)
- Triple-Quadrupol-ICP-MS (inaktiv und radioaktiv)
- Sektorfeld-ICP-MS (radioaktiv)
- GC-MS (Triple-Quadrupol, inaktiv)
Cooperations in the field of AMS:
VERA (Wien)
ETH (Zürich)
For the analysis of radioactive samples see also nuclear spectrometry and routine methods.
Contact:
|
+49 721 608 22233 |
+49 721 608 24747 |
Routine methods
At INE a pool of routine analytical methods is available.
- ion chromatography (cations and anions, radioactive and inactive samples)
- ICP-OES (radioactive and inactive)
- Flame-AES (only inactive samples)
- WDXRF
- organic and inorganic carbon (TOC, DOC, NPOC, radioactive and inactive)
- surface determination after BET (inactive)
- column separations
- digestions (conventional or microwave)
- volumetric analysis
- gravimetric analysis
For the analysis of radioactive samples see also mass spectrometry and nuclear spectrometry .
Contact:
|
+49 721 608 24747 |
Hyphenated fractionation-based methods as tools for the actinide speciation in solution
Actinides speciation in solution is necessary to elucidate their environmental behaviour. Speciation information can be obtained by sample fractionation and subsequent sensitive detections. The coupling of Inductively Coupled Plasma Mass Spectrometry (ICP-MS) with separation procedures like the Asymmetric Flow Field-Flow Fractionation (AsFlFFF) or the Capillary Electrophoresis (CE) allows sample fractionation and detection of a variety of elements at low concentrations relevant for environmental purposes.
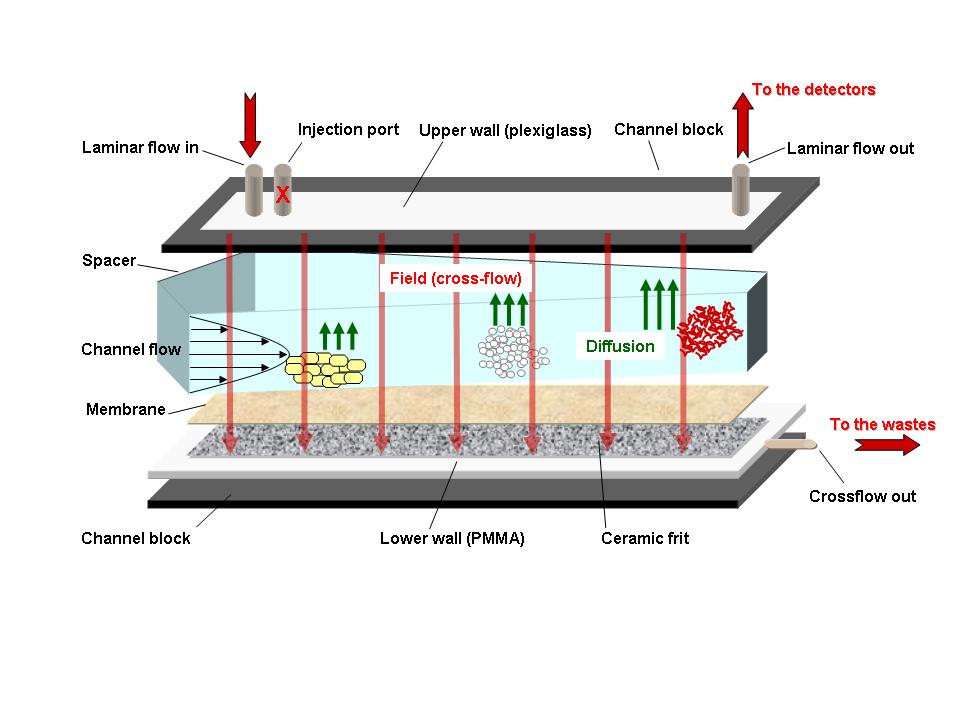
AsFFFF offers the possibility to separate colloidal matter ranging from <1 nm up to 100 µm. Colloid size fractionation takes place in a thin ribbon-like flow channel by applying a cross flow perpendicular to the channel flow (Scheme 1). At INE, the AsFlFFF is coupled on-line with a UV-Visible spectrophotometer, a Laser Light Scattering (LLS) detector and a Laser-Induced Breakdown Detection system (LIBD) or an ICP-MS. The LIBD is characterized by its particularly high sensitivity while the coupling with ICP-MS allows to gain insight into the elemental composition of colloids and notably the elucidation of trace metal interactions with colloids. The results obtained provide semi quantitative view into the trace elements distribution in colloidal systems. This flexible colloid characterization method has already been applied successfully at INE to the examination of inorganic particles (e.g. clays, iron oxide/hydroxides), humic substances, hydrocolloids in general and synthetic nanoparticles like quantum dots.
CE-ICP-MS (Figure 2) use for actinide speciation at low concentration is a well established technique which does not disturb the redox species ratio during the measurement. It has been recently applied at INE (picture) to study the Pu(IV) hydrolysis, the redox speciation of arsenic (As(III)/(V)) and selenium (Se(IV)/(VI)) and to the separation of uranium species (U(IV)/(VI)).
Complementary informations are obtained by using additional spectroscopic (EXAFS, TRLFS) or imaging (AFM, SEM, TEM, STXM) techniques.
Contact:
+49 721 608 24939