Actinide coordination and extraction chemistry
The coordination chemistry of actinides plays a major role in various areas such as the development of extraction processes for the separation of actinides and in some aspects of safety research. Against this background, we perform fundamental spectroscopic investigations and extraction studies on the coordination of actinides and lanthanides with inorganic and organic ligands. The aim is to gain in-depth insight into the bonding states, structures and stabilities of the complexes formed.
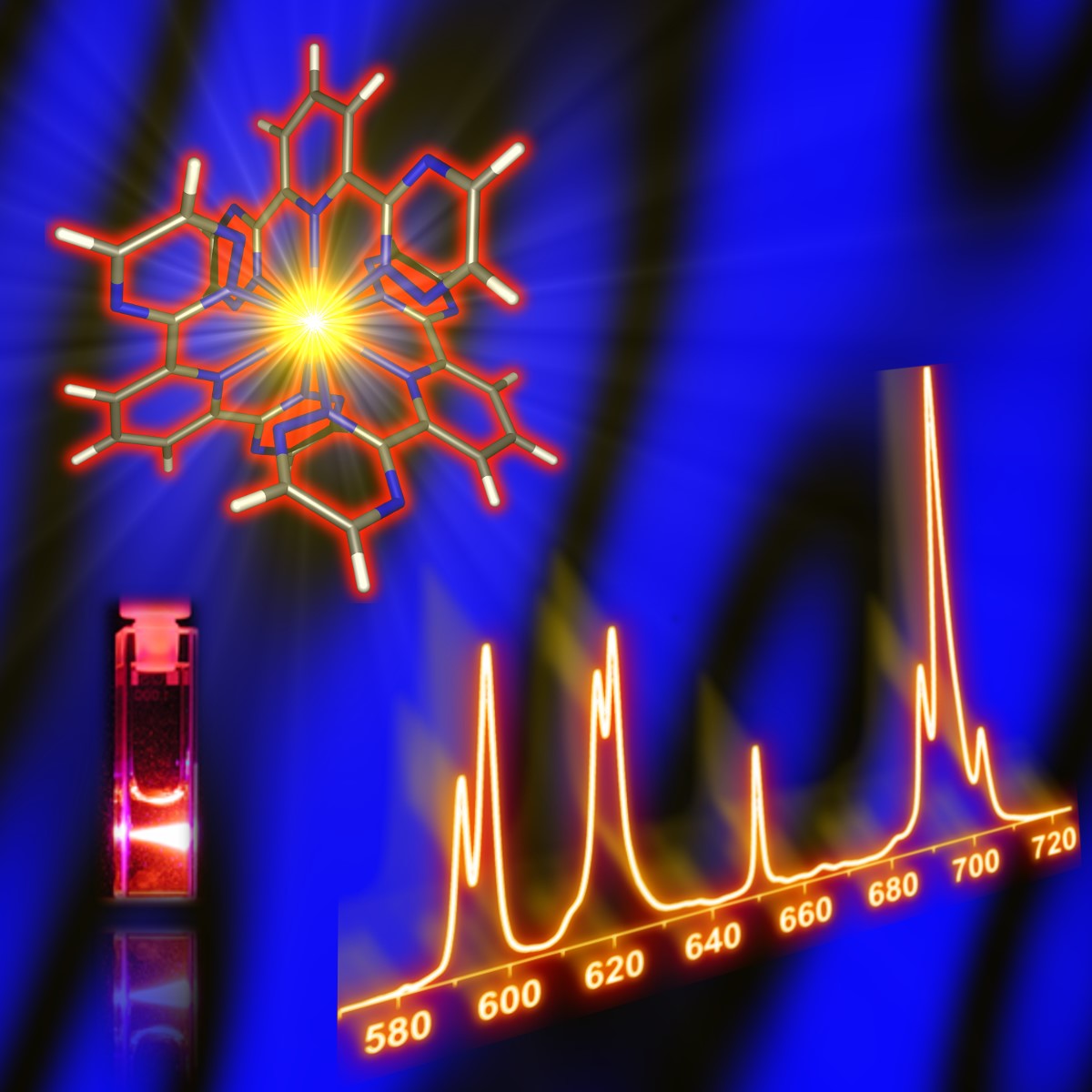
A particular focus is on so-called soft donor ligands, coordinating via ‘soft’ donor atoms such as nitrogen or sulphur. Unlike oxygen-based ligands, their affinity for actinides is higher than for the chemically similar lanthanides. This difference provides the basis for a variety of solvent extraction-based separation processes that are being developed worldwide. For example, various aromatic nitrogen heterocyclic compounds (e.g. bis-trazinyl-(bi)pyridines, bis-trazolyl-(bi)pyridines) have been developed and tested as hydrophobic extracting agents or hydrophilic complexing agents for actinide-lanthanide separations. In this context, the bis-triazinyl pyridines used for the first time at INE (see Z. Kolarik et al., Solvent Extraction & Ion Exchange 1999, 17, 23) represented an internationally recognised breakthrough.
Based on our extensive expertise in the field of solvent extraction chemistry and the development of solvent extraction processes, we have also developed a system for the extractive separation of radiocaesium from saline solutions.
Our research combines state-of-the-art spectroscopic methods, including nuclear magnetic resonance spectroscopy (NMR), time-resolved laser fluorescence spectroscopy (TRLFS) and X-ray-based techniques, with theoretical approaches. This interdisciplinary approach allows the comparative characterisation of actinide, lanthanide and other metal complexes and the precise quantification of the subtle differences in the interactions between metal ions and ligands, which are crucial for the processes under investigation.
The work is carried out in close co-operation with the University of Heidelberg (working group of Prof. P. J. Panak) and is integrated into various national and international co-operations and projects.
Contact:
| Prof. Dr. Petra Panak | Dr. Andreas Geist | Dr. Thomas Sittel |
| +49 721 608 24469 | +49 721 608 26249 | +49 721 608 24652 |