Laser Spectroscopy
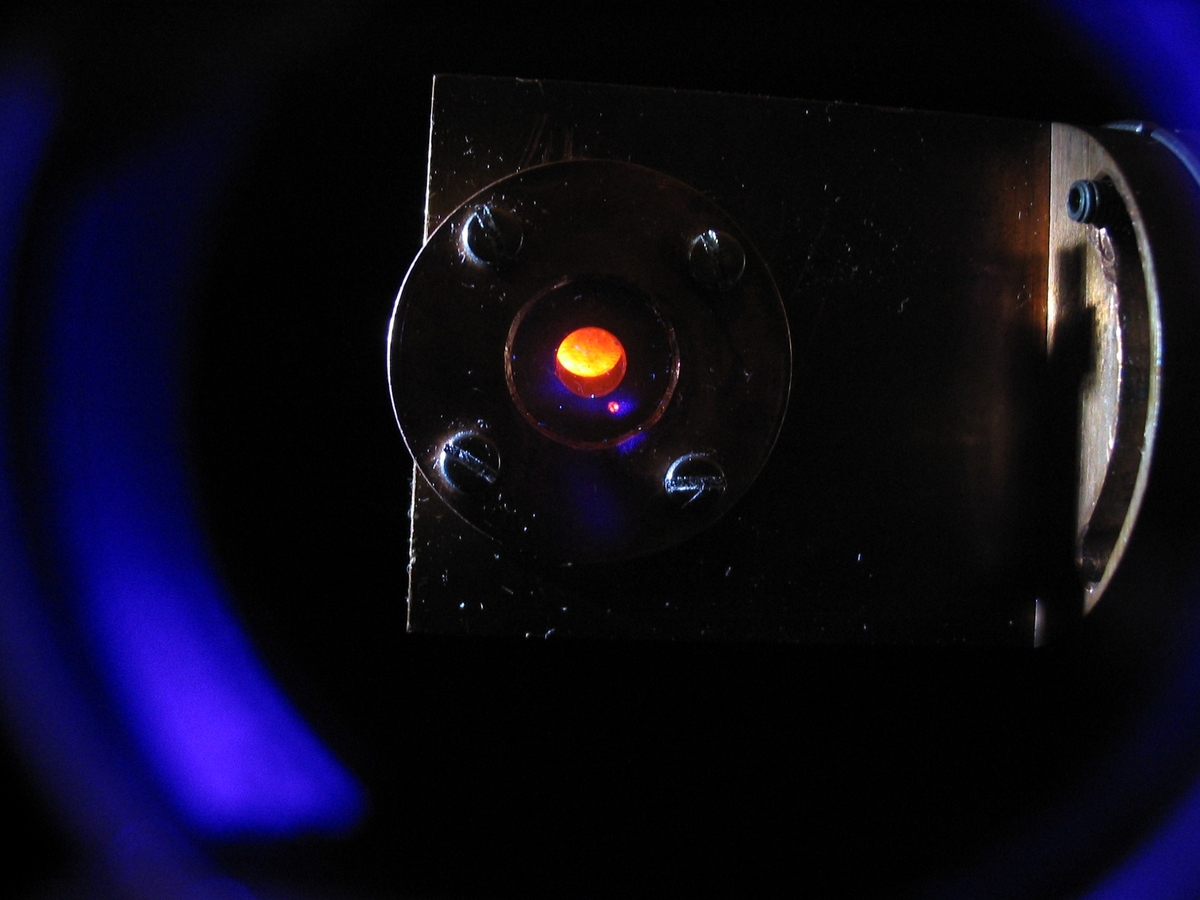
Since the development of the most common laser types in the late 1960’s, the use of laser based methods in different fields of spectroscopy has been continuously growing. Besides a minimum of sample preparation requirements, most of these methods feature superior detection sensitivity. Time-resolved laser fluorescence spectroscopy (TRLFS) is a method routinely applied at INE for the chemical speciation of lanthanide and actinide cations (e.g., Eu(III), U(IV), U(VI), Am(III) or Cm(III)) at trace level concentrations down to 1.0E+08 atoms in the sample – both in solid and liquid phase. Following excitation by pulsed laser light, luminescence decay kinetics within ns to ms time scales can be recorded. From the luminescence decay lifetime of aqueous complexes the number of water molecules in the first coordination sphere is derived, allowing to obtain structural information of the complex. In conjunction with the shift or splitting of the emission peaks, different lanthanide and actinide species present in the sample are discernible.
Contact :
Dr. Andrej Skerencak-Frech Tobias Hippel
+49 721 608 26024 +49 721 608 29333
Laser-Induced Breakdown-Detection (LIBD)
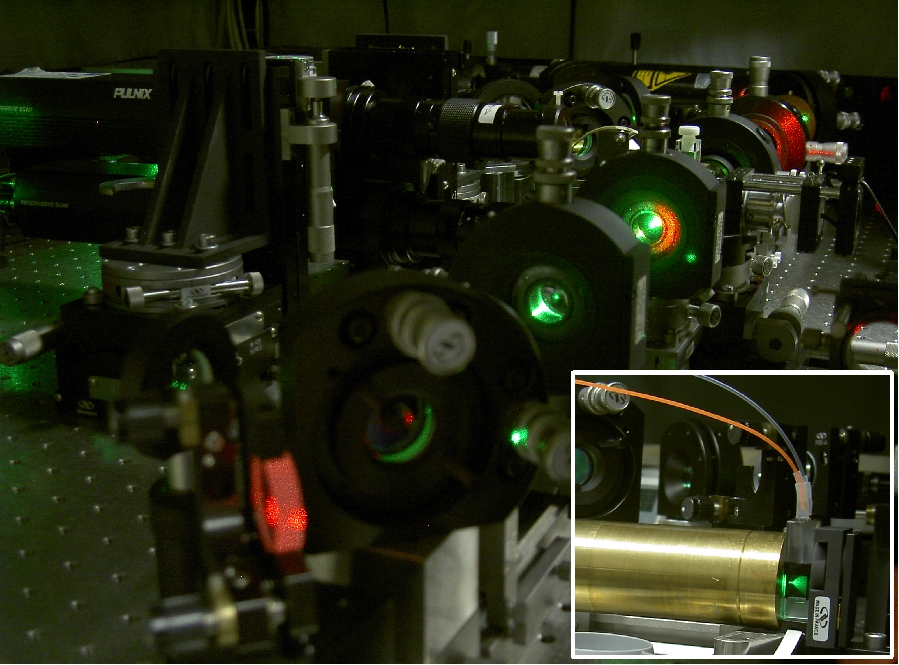
The Laser-Induced Breakdown-Detection (LIBD) has been developed for the direct, highly sensitive quantification of nanoparticles in liquids (aquatic colloids). Compared to methods based on light scattering LIBD is several orders of magnitude more sensitive especially for particles < 100 nm (Limit of detection ca. 10.000 part/ml).
The method is based on the generation of a dielectric breakdown (plasma generation) in the focus of a pulsed high-energy laser beam. As the intensity-threshold for a breakdown ignition is lower in the solid-state than in liquids, breakdown events can be initiated by particles in the focal region at suitable laser pulse-energy. This plasma-emission and the succeeding pressure-wave are detected with a computer-based image-detection system or a photoacoustic sensor, respectively. Using a calibration obtained from monodisperse reference spheres, mean size and concentration of colloids are derived. By use of the acoustic detection also the size distribution between 15 nm and 1000 nm can be measured.
Applications of LIBD are twofold: (A) The first are investigations of true colloids, i.e in the present case colloids composed of radionuclides. Formation, stability and aggregation behavior of zirconium, thorium, plutonium and curium colloids are examined in order to obtain thermodynamic solubility data, highly relevant for the assessment of chemical reactions in the near field of a repository.
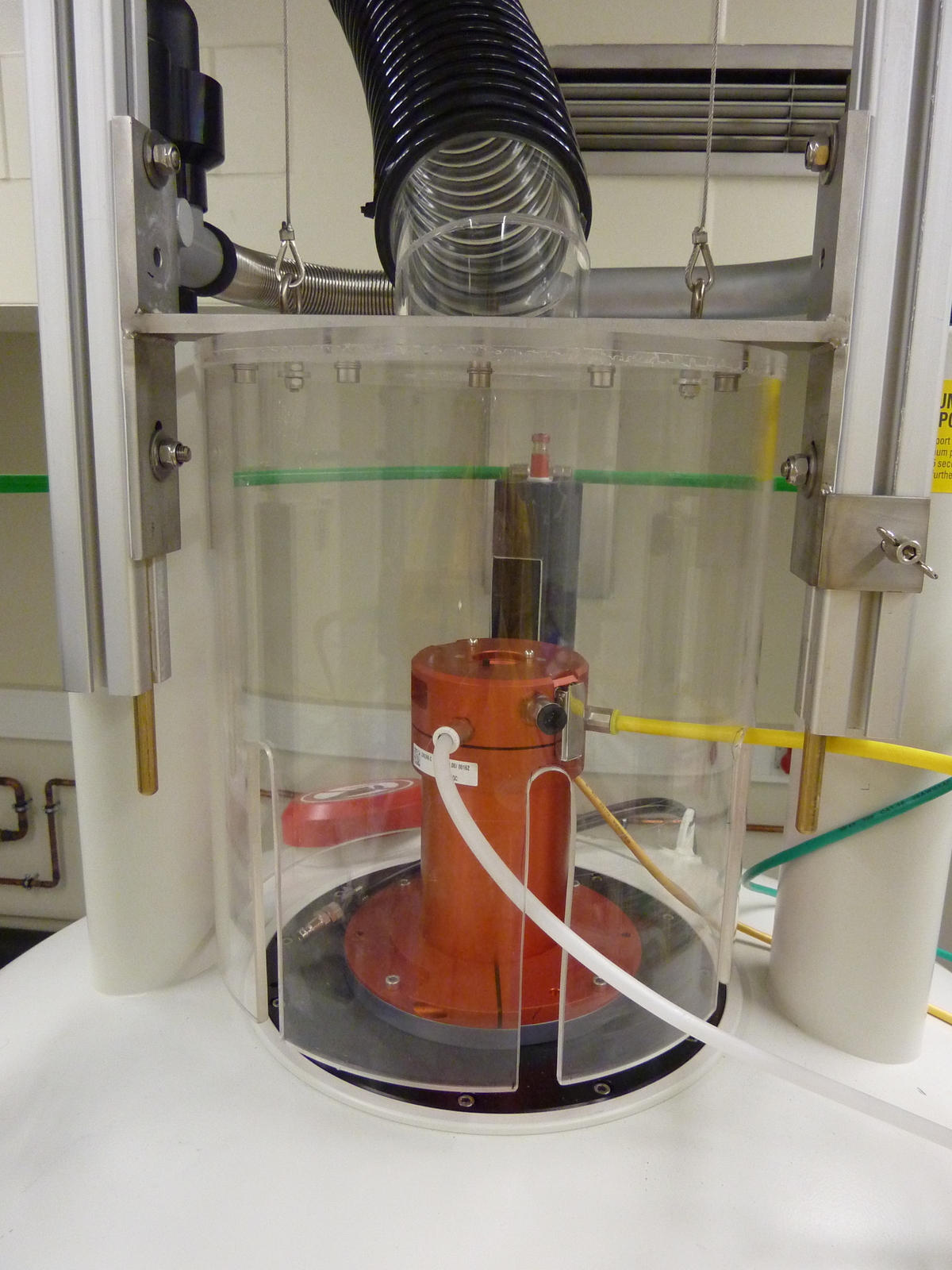
(B) Pseudocolloids, i.e. radionucludes attached to colloids of natural origin may lead to unwanted colloid mediated radionuclide migration. For the geochemical characterization of groundwater under in-situ conditions a mobile LIBD instrumentation is used. Investigations are performed under real disposal conditions with an optical detection cell designed for hydrostatic pressures up to 50 bar.
Contact:
+49 721 608 29333
NMR spectroscopy
NMR spectroscopy allows for a facile, nondestructive access to the determination of constitution, configuration and conformation of molecules in solution as well as in solid state and represents the most prominent method for structure elucidation of organic compounds. Besides the structure determination one can easily address dynamic process in a wide range of time constants from to microsecond to the seconds range.
In order to understand the formation of complexes on a molecular level, a deeper insight into the electronic configuration of partitioning relevant ligands is necessary. Therefore appropriate sensors inside the molecule have to be identified, reporting of the changes in the electronic structure when complex formation occurs. As NMR spectroscopy finally measures the electronic surrounding of the nuclei under investigation, it is very well suited to give the necessary answers. Measurements of the relaxation properties as well as diffusion-ordered spectroscopy allow for determining different parameters of the complex formation process and the final size of the complex.
The influence on NMR-active nuclei is restricted on a radius of approximately 10 A through space and only a view bonds in a molecule. Therefore NMR spectroscopy is ideally suited to investigate amorphous systems. Experiments on glasses as well as on colloidal systems formed under repository relevant conditions allow for addressing questions of retardation or mobilization of actinides in those systems. For those measurements a variety of NMR-active isotopes, like 71Ga, 27Al, 11B, 33S, 17O, 29Si, 95Mo are under investigation in solutions as well as in solid state.
The NMR instrumentation at KIT-INE is installed in the controlled area. Together with suitable security measures investigations on radioactive samples in solution are possible. Therefore, the NMR instrumentation at KIT-INE is one of the few spectrometers in the world where it is possible to perform experiments on systems in the field of repository and security research of high level nuclear waste disposal.
Contact:
N.N.